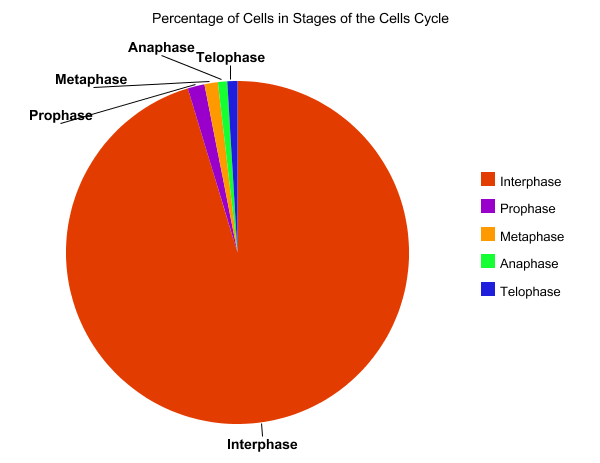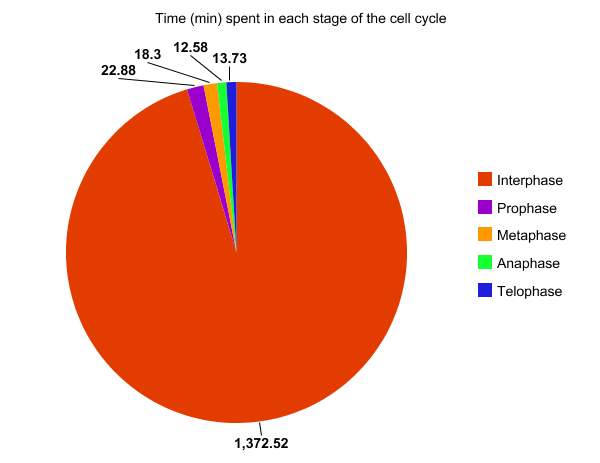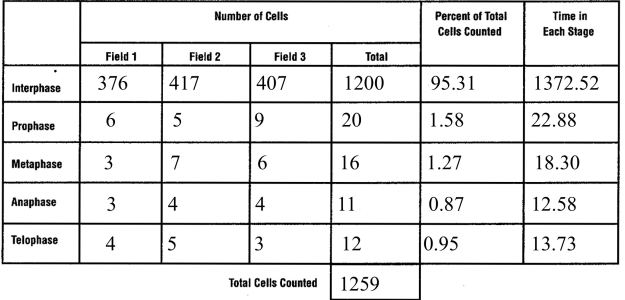
This past week, I had the privilege to talk with and learn from a survivor of cancer. My interviewee was a seventy-seven year old man who had prostate cancer. While he is in remission, I could tell this was still a very sensitive and painful topic to discuss. Two years prior, he had joined a new health care system, and had gone to a routine medical checkup for the first time in a few years. On a routine blood test, a PSA level was checked, and it was determined that it was elevated. The PSA, or prostate specific antigen was eleven. He told me this meant the chance of him having prostrate cancer was over fifty percent. He then underwent a CT Scan, a bone scan, and eventually a prostate biopsy. The prostate biopsy “was uncomfortable,” he remarked. He joked, “he had no symptoms before the biopsy, only after.” After it was discovered that he had cancer, he alternated between denial and “I am going to beat this” attitude. Admittedly, he told me, that he was scared of the dreaded c-word. After he was diagnosed, he told me that he was given a choice; surgery, or radiation and hormone therapy. He was reluctant to have surgery in fear of lifelong incontinence, so he chose hormone therapy with radiation. My survivor received external beam radiation, five days a week for a nine week period and he started a hormone therapy called Lupron therapy. He told me that the radiation therapy targeted the cancer cells in his prostate, and the Lupron stopped the release of testosterone. This slows or stops the growth of cells including his cancer cells which are testosterone dependent. Initially, his travel plans with his wife had to be altered to allow for the nine weeks of radiation therapy. He associated the treatment with his extreme fatigue, which prohibited him from enjoying his everyday lifestyle. My interviewee said that he was grateful for his wife in helping him, and for all the support from his family and friends, while struggling with not wanting to be a burden to them. He grew closer to a friend who had previously survived prostate cancer, but did not feel comfortable talking about it to most of his other friends. He focused more on the present tense than the future, enjoying each moment. Most of his difficultly centered upon the “what ifs” and struggling to deal with the possibility of the worst possible outcome. In regard to addressing common misconceptions about cancer, he replied, “people believe that you have no voice in your options for treatment. However, the most important thing is to become educated in your treatment options, and discuss them openly with your physician. The goal is to work with your doctor, “not follow blindly.” He shared personal advice as well: “appreciate every day; allow yourself to be inspired and inspire others. No matter what circumstance you are in, keep a positive outlook on the future and don’t dwell on the ‘what-ifs.'”
After talking with a cancer patient, I developed a strong admiration for all of those who endure the hardships of cancer. His views on life drastically changed mine, reminding me to focus on the present and not dwell on the past, or the future. Reading the text book and classroom discussions gave me a third party detailed view on cancer, but actually speaking to somebody who had experienced cancer firsthand deepened both my knowledge and perception of cancer, as well as being an august experience. I am grateful to my interviewee for all that he shared with me, and I will remember it forever.
About Prostate Cancer
According to E.O. Wilson’s Life on Earth cancer is “a disease in which abnormal cells divide uncontrollably and invade other tissues. A cancer can occur anywhere in the human body, and then spread to other tissues via metastasis.
My cancer patient developed prostate cancer, which affects the prostate gland in males. The prostate gland is a part of the male reproductive system located between the bladder and the rectum. Prostate cancer, if it becomes metastatic, can spread throughout the body, even affecting vital organs such as the liver, lung, brain and heart. Luckily, my interviewee’s cancer did not become metastatic, and was contained to the prostate.
The cause of changes that lead to prostate cancer is not known by medical professionals, however, medical researchers now understand the changes in DNA that can create cancerous prostate cells.
Contrary to popular belief, genetics are only responsible for approximately 5-10% of all prostate cancers. In these cases, genetic inheritance of three subsets of gene mutations lead to a higher rate of prostate cancer.
One of these gene mutations is RNASEL, derived from HPC1. HPC1 kills tumors when they appear in the prostate glands. The mutation of RNASEL allows the irregular cells to last longer, increasing the chance of developing prostate cancer.
Another two genes that can harmfully mutate include BRCA1 and BRCA2. These genes suppress tumors by fixing mistakes in DNA synthesis. Although mutations in these genes are uncommon, they account for a number of prostate cancers, as well as breast and ovarian cancers.
MSH2 and MLH1 are another two genes that correct irregularities in DNA. Men who inherit mutations in this gene develop the condition Lynch syndrome, also called hereditary nonpolyposis colorectal cancer (HNPCC). Men with Lynch syndrome are at a higher risk at developing prostate, colorectal, small intestine, stomach, gallbladder, and brain cancers.
In addition to inherited genes that may cause cancer, some scientists hypothesize that DNA mutations or high production of substances can attribute to increased risk of developing cancer. Increased androgen level, such as large amounts of testosterone, have been proven to promote cell growth, perhaps contributing to the cell growth of cancerous cells in the prostate.
Another hormone, insulin-like growth factor-1 (IGF-1), perhaps can correlate to an increased risk of developing prostate cancer. IGF-1 is another hormone that regulates cell growth that some studies have related to cancer growth, however, additional studies are yet to connect IGF-1 and prostate cancer.
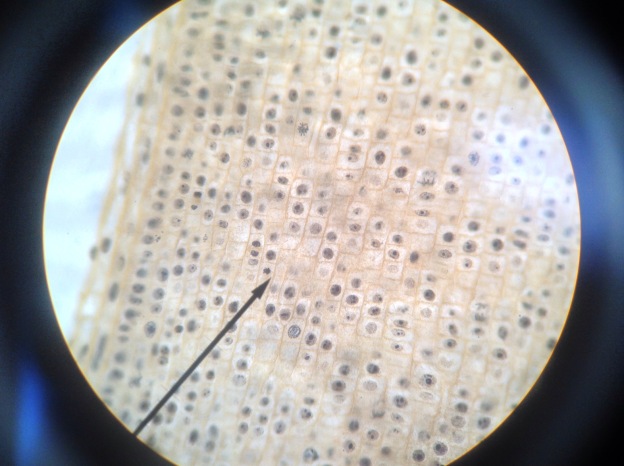
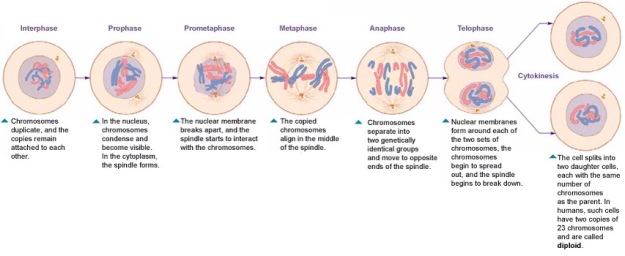
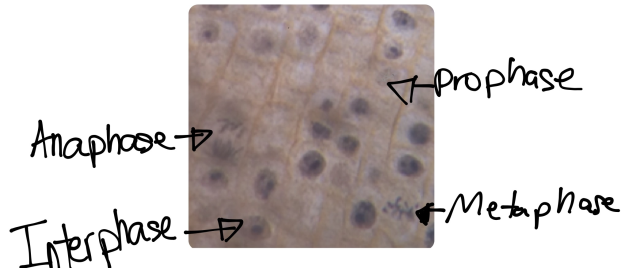
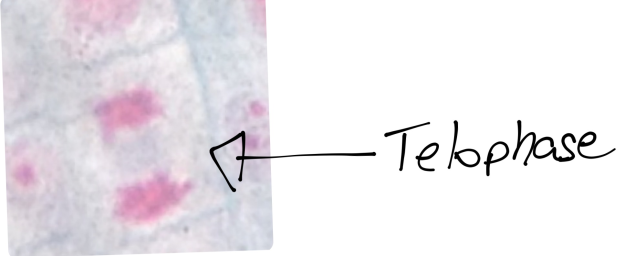
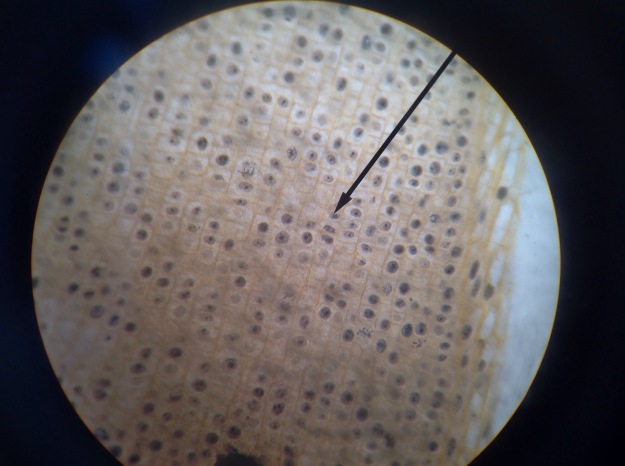
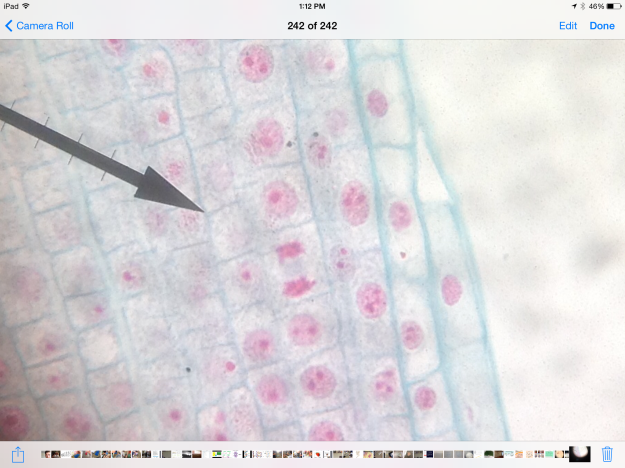
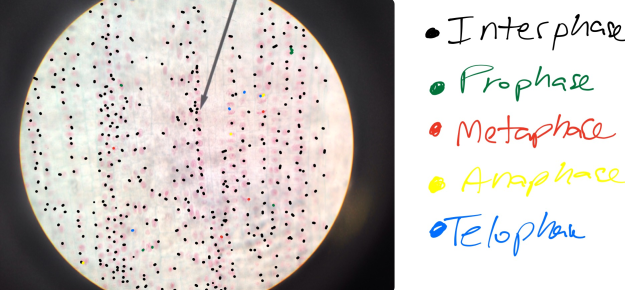
In prostate cancers, the cell cycle plays an important role. When DNA replicates during the S stage of interphase, mutations in genes described above can pass through the cell cycle, producing new cancerous cells, which can then uncontrollably divide as tumors. Other proteins that interact with cells during different stages of the cell cycle affect, modulating ARs. These include “G0 (RB), G1 to S phase (cyclin D1, cyclin E, Cdk6), or G2 (Cdk1)” (Balk-Knudsen). Errors in the modulation of ARs could possibly cause a cancer to develop.
To treat prostate cancer, medical professionals use many techniques. The patient usually can consider which path to take, dependent upon the patient’s age and life span, other health problems, the stage of the cancer, and medical opinions.
Patients with stage one prostate cancer are recommended to undergo “active surveillance”, and treatments include radiation therapy involving either external beams or brachytherapy, and radical prostatectomy.
Patients with stage two prostate cancer have the same treatment options as stage one prostate cancer patients, however, a majority of radiation therapies also include hormone therapy as well.
Patients with stage three prostate cancer usually also have the same options as stage two patients, but it is recommended by medical professionals to have radiation, then a radical prostatectomy.
Finally, patients with stage four prostate cancer unfortunately usually cannot partake in the standard cancer treatments. Treatment for stage four patients include hormone therapy, surgery to remove pain in the bladder or rectum. Bone metastatic development treatment, such as denosumab, a drug also used to cure osteoporosis, or “radiopharmaceutical such as strontium-89, samarium-153 or radium-223.” (Cancer.org)
As we do not know a definite cause for prostate cancer, there are not strong evidence of prevention, however many other ways to prevent cancers remain true for prostate cancer.
The American Cancer Society suggests that one eats 2 1/2 cups of assorted fruits and vegetables daily, one maintains a healthy weight and diet as well. Additional studies have shown that some medicines can also decrease the rate of developing prostate cancer. Early studies show that supplements of Vitamin E and selenium lower the rate of prostate cancer in males. Furthermore, “5-alpha reductase inhibitors [treat]!prostatic hyperplasia (BPH), a non-cancerous growth of the prostate” (cancer.org) These medicines include Finasteride, marketed as Proscar, and Dutasteride marketed as Avodart may decrease the risk of developing prostate cancer. Other studies suggest that prolonged aspirin intake could also decrease the rate of prostate cancer, but further studies are required.
In 2014, it is estimated that another 233,000 men in the U.S. will develop prostate cancer, and approximately 1,112,000 people will be diagnosed in the world. Of these numbers, 29,480 men in the U.S. Will die of prostate cancer.
Physicians whom help treat prostate cancer include:
–Urologists, or a surgeon that specializes in surgery of the urinary tract and the male reproductive system.
–Radiation oncologists, a physician who treat cancer with radiation therapy, and
–Medical oncologists, a doctor who treats cancer with chemotherapy or hormone therapy.
Sources
National Cancer Institute
http://www.cancer.gov/cancertopics/types/prostate
National Cancer Association
http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-treating-general-info
http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-treating-by-stage
http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-prevention
http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-what-causes
World Cancer Research Foundation
http://www.wcrf.org/int/cancer-facts-figures/worldwide-data
Genetics Home Reference
http://ghr.nlm.nih.gov/condition/lynch-syndrome
Balk, Steven and Knudsen, Karen : National Center For Biotechnology Information
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2254330/#__sec6title