In this lab, we studied the osmosis of water through the semi-permeable membrane of an egg. Osmosis occurs when a solvent moves from an area of high concentration to one of low concentration via a semi-permeable membrane. To illustrate the process of osmosis, we dissolved the eggshell via an acid-base reaction by combining calcium carbonate (a base) and acetic acid, and we then placed the denuded egg in water. The amount of water that passed through the semipermeable membrane was determined by weighing the egg at five minute intervals. We concluded that the egg membrane is semi-permeable, and we determined that the weight of the egg increased by approximately 1.6% of its original mass every five minutes.
In this experiment, we observed the process of osmosis through the semi-permeable membranes of a chicken egg. The semi-permeable membrane is located under the hard calcium carbonate shell, as illustrated in Figure 1.
Figure 1
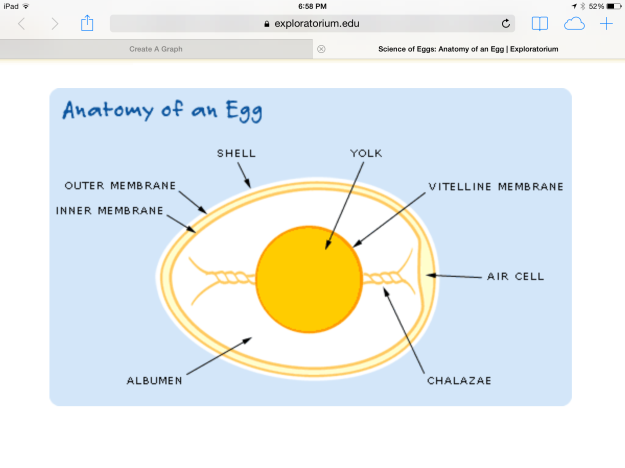
The outer shell of a chicken egg is composed of 94-97 percent CaCO3. We first dissolved the outer shell by means of an acid-base reaction with vinegar, which contains acetic acid. When calcium carbonate mixes with acetic acid (CH3CO2H) the following chemical reaction occurs: Initially, the carbonate (CO3–) part of calcium carbonate is protonated by acetic acid to make carbonic acid (H2CO3),and the calcium and acetate form calcium acetate.
2 CH3COOH + CaCO3 = H2CO3 + Ca(CH3COO)2.
Next, the carbonic acid breaks down to form carbon dioxide and water.
H2CO3 = H2O + CO2.
The overall reaction can be written as the sum of two reactions:
2 CH3COOH + CaCO3 = H2O + CO2 + Ca(CH3COO)2.
After the egg shells are dissolved, the egg’s outermost layer is composed of two semi-permeable membranes. These membranes play a vital role in the development of a chick by allowing air and moisture to pass through to the developing embryo. The membranes are partially made of keratin, and they provide the embryo with a defense against bacterial invasion.
The de-shelled eggs were placed in water in order to simulate the behavior of a cell, which is also surrounded by a semi-permeable membrane, when placed into a hypotonic solution. We predicted that water would flow by osmosis through the semi-permeable membranes down its concentration gradient. Since water is a relatively hypotonic solution, which means that it has a lower concentration of solute when compared to the interior of the egg, water flows from an area of low solute concentration to an area of high solute concentration: into the egg. The absorption of water by the egg can be measured by weighing the egg and observing an increase in circumference.
In this lab, we used the following materials:
-3 eggs of relatively the same size
-white vinegar (apple cider or rice wine vinegar will work too)
-large beaker
-three large plastic cups
-triple beam balance scale
-plastic tray
-300ml of distilled water
-timer
Preparing the eggs:
1.I placed the eggs in the beaker gently without cracking the shell. I covered the eggs in the vinegar and let them sit overnight.
2. After the eggs were soaked, I removed them from the vinegar solution, and I gently rubbed off the white calcium acetate from the egg.
Starting the experiment:
1. I filled a cup with 100 ml of distilled water.
2. I marked each egg with a number (1-3), and I weighed egg 1with the triple beam balance, after placing the egg in the plastic tray.
3. I added egg 1 to the cup.
4. I let the egg sit in the water for five minutes, and I then weighed it again.
5. I repeated steps 1-4 using eggs 2 and 3.
Measuring the rate of osmosis:
1. I weighed egg 1 in the plastic tray after five minutes in the water bath, and I returned it to the bath as soon as possible.
2. After a second five minute interval, I re-weighed the egg 1 and returned it to the water for a third five minute period.
3. I removed egg 1 and weighed it a final time.
4. I repeated steps 1-3 for eggs 2 and 3.
5. I recorded my data in the table.
Note: When recording data, I accounted for the weight of the plastic tray.
Figure 2

Figure 2 illustrates the egg’s CaCO3 shell being dissolved by the surrounding vinegar. Carbon dioxide bubbles surround the egg, because CO2 is a product of this reaction as described previously.
Figure 3

Figure 3 depicts the egg after the shell has disintegrated. A layer of calcium acetate, another product of the reaction, is visible on the egg. After the shell dissolved, the egg became rubbery to the touch.
Figure 4

Figures 4 and 5 depict the eggs being weighed on the triple beam balance. I found that the weight of egg 1 increased from 71.5 g to 74 g after five minutes, it remained at 74 g after ten minutes, and it increased to 75 g after 15 minutes. The weight of egg 2 increased from 76.5 g to 78 g, 79g, and 80 g after five, ten and fifteen minute intervals respectively. Finally, the weight of egg 3 increased from 86.8 g to 88 g, 89.3 g, and 91g after sequential five minute immersions.
Figure 6
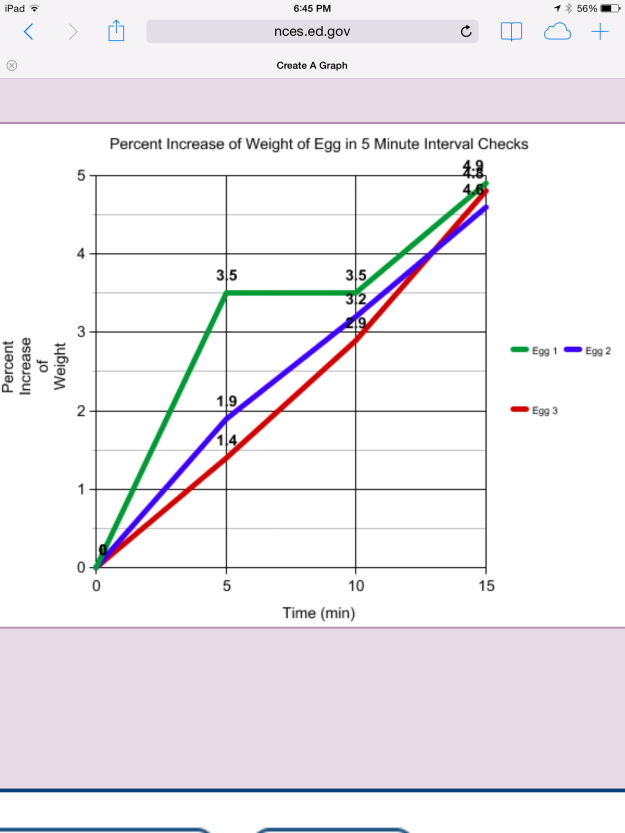
The graph in Figure 6 illustrates the increase in weight of each egg (expressed as percent weight gain) during the time they were immersed in distilled water.
Figure 7
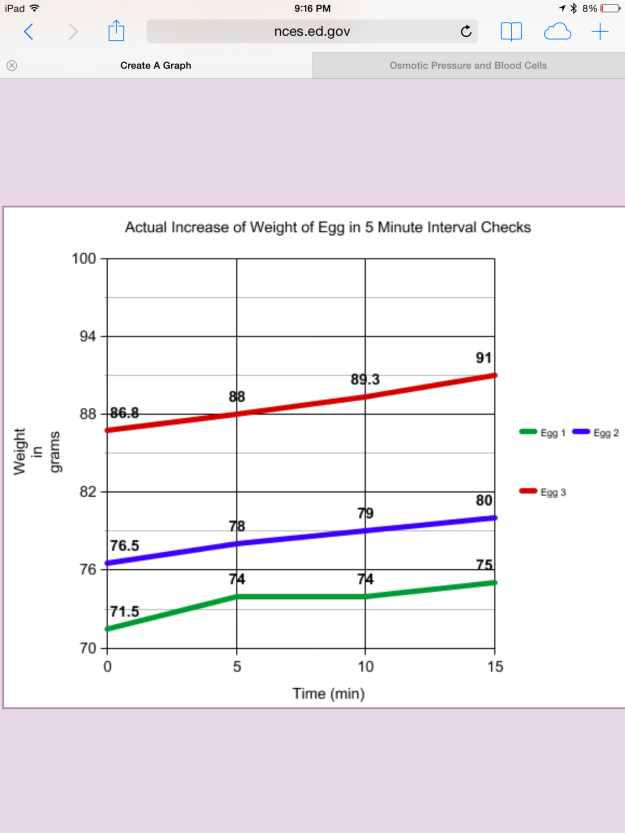
The graph in Figure 7 depicts the increase in actual weight of the eggs during the time they were immersed in distilled water. It is apparent that the weight of the eggs increased with a longer duration of immersion in water.
Figure 8
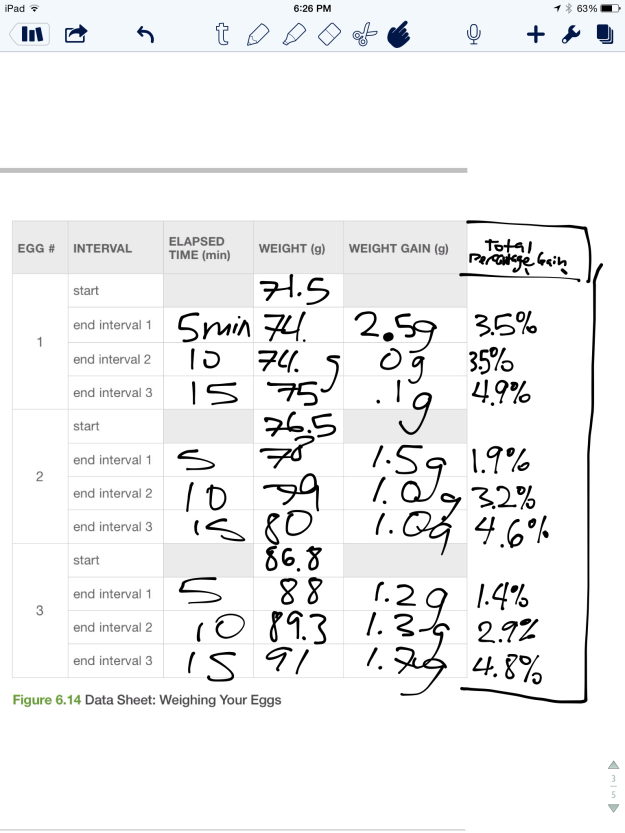
Figure 8 depicts the actual weight gain and percent increase in the weight of each egg during the experiment. As discussed previously, the weight of each egg progressively increases during the experiment, as the eggs absorb water by osmosis through the semi-permeable membranes.
Figure 9

Figure 9 depicts an egg that ruptured after the experiment was complete. At the top of the photo, the gray semipermeable membrane lies next to the egg yolk. We noted that the thickness of the egg membrane is quite small in comparison to the size of an intact egg.
Discussion Questions:
*When the eggs were placed in the vinegar, they swelled because the reaction of vinegar and the eggshell (2 CH3COOH + CaCO3 = H2O + CO2 + Ca(CH3COO)2.), results in water, which moved into the egg by osmosis.
*In the first part of the experiment, water moved into the egg. We know that water has moved into the egg, because they gained weight during the immersion in distilled water.
*Chicken egg membranes are semi-permeable because water was able to move into the egg by osmosis but no egg albumin (which is a large molecule) was able to diffuse through the membrane and out of the egg.
*It is possible to calculate an egg’s volume by measuring the amount of water displaced by the egg. If a beaker is filled with water and an egg is submerged in the water, the amount of displaced water can be measured and is equal to the volume of the egg. A change in weight indicates that more water has moved into the egg, and this would likely be accompanied by a change in volume. This is because the egg expands as water passes into it.
*Another possible experiment is to place the de-shelled egg in solutions of different tonicity (isotonic, hypertonic, and hypotonic) in order to determine the effect of those solutions on the water content of the egg. I anticipate that the egg would shrink when placed into a hypertonic solution, and an isotonic solution would have no effect of the egg. It would also be interesting to study active transport of molecules across an egg membrane.
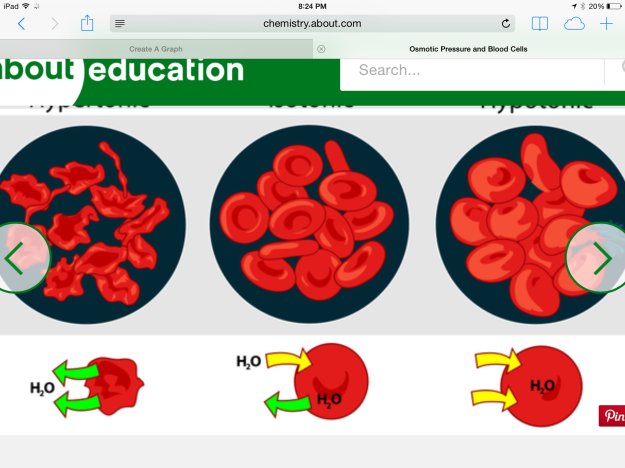
*Saline solution is used to expand blood volume, as it is isotonic. Hypotonic distilled water enters red blood cells via osmosis and causes them to burst open (see Figure 10).
Figure 10
In this experiment, we observed an acid base reaction, osmosis through a semipermeable membrane and the effect of a hypotonic solution on the weight of a chicken egg.
The first part of the experiment involved a chemical reaction between a base (CaCO3), which is found in egg shells, and an acid (acetic acid), which is found in vinegar.
The chemical reaction is:
2 CH3COOH + CaCO3 = H2O + CO2 + Ca(CH3COO)2.
We observed carbon dioxide bubbles, the dissolution of the hard calcium carbonate shell, and the formation of calcium acetate. We also noted that the egg became rubbery after immersion in vinegar. This may have occurred because the acid started to denature the albumin found in egg whites. Denaturation of proteins can occur through exposure to acids, bases or high temperature.
The next part of our experiment was to observe the osmosis of water through the semipermeable membranes of the egg, which lie under the hard shell. These membranes are important in the development of the embryo because they are permeable to water and air, and they provide a barrier to dust and bacteria. After we soaked the egg in water (a hypotonic solution), the egg began to enlarge. We weighed the egg at five minute intervals, and we noted an average increase in weight of 2.2% over the first five minutes, then an average increase of 0.8% over the next five minutes, then an average increase of 1.2% during the final five minutes (see Figure 7). The increase may have slowed as the experiment progressed because the concentration gradient decreased over time.
Next, we observed the effect of the tonicity of the solution on the egg as a model for the reaction of a cell to its environment (or a bathing solution). The egg swelled because the hypotonic solution resulted in an osmotic gradient that caused water to flow from a low concentration of solute to a high concentration. In this case, the high solute concentration was provided by egg proteins.
If a cell is in an isotonic environment (the external osmolarity is equal to the internal osmolarity), the cell volume remains unchanged. If a cell is in a hypertonic environment (the external osmolarity is higher than the internal osmolarity), the cell will shrink. Finally, if a cell is in a hypotonic environment (the external osmolarity is less than the internal osmolarity), the cell will swell.
The relative osmolarity of a solution is determined by the number of solute particles, and not by type of particles. An increase in temperature will speed up the motion of molecules and will also increase the rate of diffusion or osmosis. Although diffusion occurs in both directions, the overall net movement will be down the concentration gradient.
If we were to repeat this experiment, I would use solutions with different osmolarity, and I would vary the temperature to see if the rate of osmosis would be affected. Finally, I would study the egg membrane’s ability to let air and water flow osmotically while preventing the movement of bacteria and large molecules. It is possible that eggs could be analyzed for an intact shell and cell membranes to decrease the possibility of eating eggs that are contaminated with salmonella and other disease causing organisms. If one could develop an inexpensive semi-permeable membrane, it might be useful in the desalination of water for use in developing countries.
https://www.exploratorium.edu/cooking/eggs/eggcomposition.html
World’s Poultry Science Journal/Volume 61/Issue 1/March 2005, pp 71-86
Van.physics.illinois.edu/qa/listing.php?id=461
imaginationstationtoledo.org/content/2011/04/how-to-make-a-naked-egg
