In this lab, we studied the diffusion of substances through a semi-permeable membrane. We found that smaller molecules like iodine and glucose were able to pass through the dialysis tubing, while larger starch molecules did not diffuse through the membrane. The pore size determined which molecules passed through the membrane. A similar phenomenon is seen in cell membranes, as the phospholipid bilayer also acts as a semi-permeable membrane that limits the passage of certain molecules into and out of the cell.
This experiment allowed me to visualize the diffusion of iodine, glucose, and starch through dialysis tubing. Diffusion is the process by which material moves from regions of higher concentration to areas of lower concentration. Diffusion is important to living cells, as it is one mechanism for moving substances into or out of the cell through the phospholipid bilayer. Diffusion is also used by physicians to treat kidney failure with dialysis.
We used Lugol’s iodine in the experiment for three reasons: it is colored, so it is visible to the naked eye; its molecules are small in size, which allows them to pass through the membrane; and molecular iodine is not soluble in water, but it forms a triiodide molecule (and becomes water soluble) when potassium iodide is added.
Formation of a triiodide:
We used glucose because it is a small molecule and it is easy to measure it in solution with a glucose strip. In addition, we used starch because it is a large molecule and reacts with Lugol’s iodine to cause a color change.
We used the following materials to demonstrate diffusion through a dialysis membrane:
-2 250ml beakers
-2 15cm pieces of dialysis tubing
-Glucose test strips
-Solution of Lugol’s iodine in a dropper bottle
-15ml glucose solution
-15 ml soluble starch solution
-distilled water
Preparation of the starch beaker:
1. I poured enough distilled water (about 125ml) to fill half of the beaker.
2. I tied off one end of a piece of dialysis tubing and added the starch solution.
3. I tied off the other end of the tubing and rinsed the outside with water.
4. I placed the tubing into the beaker of distilled water.
5. I added enough Lugol’s iodine to change the color of the water (20 drops).
6. I waited 20 minutes before recording my results.
Preparation of the glucose beaker:
1. I poured enough distilled water (about 125ml) to fill half of the beaker.
2. I tied off one end of a piece of dialysis tubing and added the glucose solution.
3. I tied off the other end and rinsed the tubing as discussed above.
4. I placed the tubing into the beaker of distilled water.
5. I recorded the results 20 minutes later.
Testing the solutions:
1. In the presence of starch, the Lugol’s iodine will change the solution into a deep blue or black color.
2. In the presence of glucose, a glucose test strip will change color when it is placed into the solution.
Diffusion of Lugol’s iodine and starch:
Figure 1

Figure 1 illustrates the assessment of starch and iodine diffusion through a semi-permeable membrane.
Figure 2
Figure 2 illustrates the addition of iodine to the beaker, which made the distilled water turn a light shade of yellow. The starch solution is inside the dialysis tubing.
Figure 3
Figure 3 illustrates the iodine/starch experiment during monitoring to detect a color change.
Figure 4

Figure 4 illustrates the iodine-starch beaker after twenty minutes. The iodine has diffused into the dialysis tubing and reacted with the starch, which has changed the solution color to blue. It is apparent that the starch did not diffuse through the semi-permeable dialysis tubing, as the color of the solution outside the tubing remains yellow.
Diffusion of glucose:
Figure 5
Figure 5 illustrates the assessment of glucose diffusion through a semi-permeable membrane.
Figure 6
Figure 6 illustrates the glucose diffusion experiment during monitoring for twenty minutes.
Figure 7
Figure 7 illustrates Mr. Wong testing the glucose solution with test strips.
Figure 8
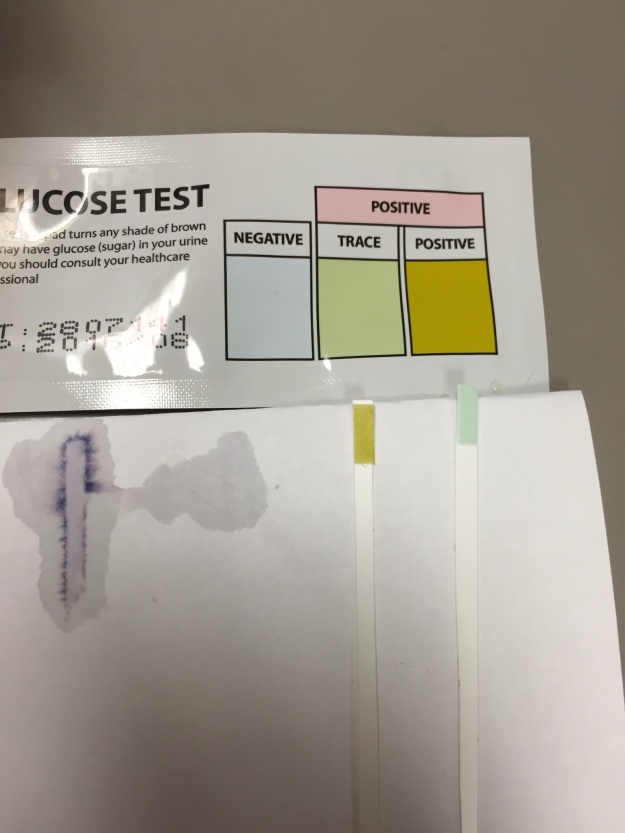
Figure 8 depicts the test strip for our beaker on the right with a distilled water control on the left. The test strip turned yellow, consistent with the presence of glucose, which showed that it diffused through the semi-permeable membrane of the tubing.
In this experiment, I explored the effect of molecule size on diffusion using a selectively permeable membrane. This process is comparable to diffusion through cell membranes, which regulates the substances that enter and exit the cell. Diffusion is caused by the random movement of molecules, and results in substances moving from regions of higher concentration to those of lower concentration.
Lugol’s iodine, which is molecular iodine combined with potassium iodide, was used in the experiment. Molecular iodine is insoluble in water, but mixing it with potassium iodide forms I3-, which is soluble in water. The chemical equation for this reaction is: I2 + I- ——> I3-
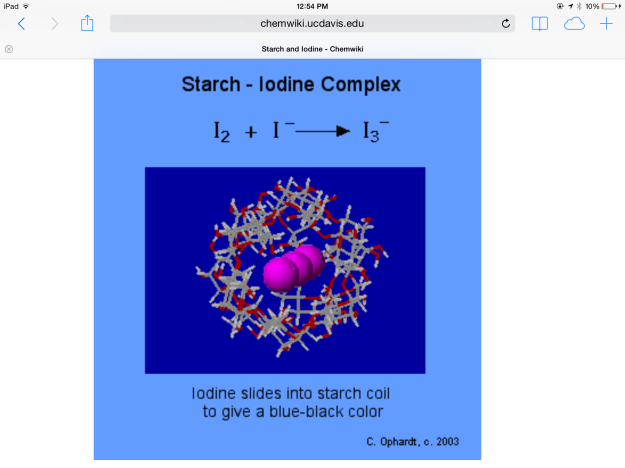
*Initially, enough Lugol’s iodine was added to the water in beaker A to give the water a yellow hue. The triiodide diffused through the semipermeable membrane and turned the soluble starch solution blue. The color change occurred because the triiodide slipped into the beta amylose coils of the starch (see Figure 9). Perhaps if the experiment were continued for a longer duration, this binding would remove the iodine ions from solution and make the solution outside the beaker lighter.
Figure 9
*The chemical test for glucose demonstrated that the glucose molecules in the dialysis tubing of beaker B diffused through the semipermeable membrane. This was confirmed by testing distilled water as a negative control, which did not change the test strip. In contrast, glucose solution was used as a positive control and changed the test strip color.
*Although we did not let the beaker sit overnight, and the only substance that we showed to diffuse is glucose, I hypothesize that water would pass through the membrane by the process of osmosis. Osmosis is the movement of water through a semipermeable membrane to form an equal water molecule concentration on both sides of the membrane.
* The starch did not pass through the membrane. This was shown when the solution inside the tubing turned blue after iodine was added due to the reaction between triiodide and starch. The solution outside the tubing remained the yellow color of the iodine, which indicated that the starch did not diffuse outside the tube. This is because the large size of the starch molecules did not permit diffusion through the dialysis membrane.
* I hypothesize that the pore diameter of the dialysis membrane is between the size of a glucose molecule and a starch molecule. This is because the glucose, iodine, and water all passed through the membrane, but the starch remained inside the tubing.
* I assume that the pore diameter of the tubing is between the size of the starch and glucose molecules. Furthermore, the pore diameter did not change, and the molecules diffused based only on size, and not because of polarity or charge.
If I were to repeat this experiment, I would make sure to clearly mark the beakers. My group accidentally placed iodine in both solutions, but we luckily caught our mistake and corrected it promptly. In addition, I would try different dialysis tubing with varied pore diameter to determine the relative size of glucose, starch, and iodine molecules. Furthermore, I would like to test other substances, including lipids and proteins, in place of starch. Finally, I would like to create an experimental membrane with an active transport mechanism in order to visualize the process of active transport, in contrast to diffusion and osmosis.
Chemwiki.ucdavis.edu